Psychedelic Week for December 4, 2022
Psilocybin case report, DEA raises production limits, long lines at Portland mushroom shop, a critique of the Breakthrough Therapies Act, final meeting of Oregon advisory board, and upcoming events.
Despite the approaching new year, there was no shortage of psychedelic news last week. In this edition of Psychedelic Week, I discuss a concerning psilocybin case report that critiques state psychedelic laws, the DEA’s increase in annual psychedelic production limits, long lines at Portland’s illegal mushroom shop, a response to the Breakthrough Therapies Act, and the final 2022 meeting of Oregon’s Psilocybin Advisory Board.
This week’s image is Dall-E 2’s interpretation of a futuristic mushroom doctor extracting data from a client.
Researchers critique Oregon’s psilocybin services
On December 1, the American Journal of Psychiatry published an article describing “A Case of Prolonged Mania, Psychosis, and Serious Depression After Psilocybin Use: Implications of Increased Psychedelic Drug Availability.” According to this case report, hours after consuming psilocybin mushrooms, a 32-year-old woman developed symptoms of mania that persisted for 3 months. As those symptoms resolved, she became severely depressed.
The case report is disturbing and should be taken seriously (one could write an entire article analyzing its details). But here, I’ll focus on the article’s characterization of state psychedelic laws, like those in Oregon and Colorado.
Understandably, the authors worry that these state laws might produce negative outcomes like the case they describe. But the adverse effects they describe would be less likely under Oregon or Colorado’s regulated programs, which require professional facilitators who could have intervened early. The article mischaracterizes these programs, which could contribute to existing misconceptions and increase the likelihood of adverse events.
According to the authors, “Oregon voters passed Measure 109, a statewide ballot initiative, to create a mental health commission tasked with developing a psilocybin-assisted psychotherapy program.” But Measure 109 did not create a mental health commission, nor did it produce a therapeutic program.
Oregon’s psilocybin services are comparable to the non-therapeutic adult use cannabis programs of many states. However, because Oregon requires people to be supervised while consuming psilocybin, its program is best described as a form of supported adult use. This distinction is important, and anyone writing about psychedelics should understand that Oregon did not legalize psilocybin for therapeutic use. In a new article, I describe The Varieties of Psychedelic Law and compare medical psychedelic programs to those intended for research and supported adult use.
The case report claims that “Oregon’s law ignores the fact that mental illnesses are medical conditions for which pharmacologic treatment historically has been and should continue to be provided by medical professionals.” But the Oregon Health Authority’s draft rules for the program prohibit psilocybin facilitators from diagnosing or treating health conditions.
The rules ban psilocybin services from being offered within healthcare facilities. Further, they prevent psilocybin facilitators from practicing the privileges of other professional licenses like medicine, nursing, or psychotherapy. This means facilitators cannot advertise or describe psilocybin as a therapeutic option. The informed consent document they will discuss with clients clarifies that psilocybin services are not approved by the Food and Drug Administration (FDA), they remain federally illegal, and they are not intended to treat health conditions. That’s a good thing. It creates a separation between Oregon’s psilocybin services, which are not intended for medical use, and FDA approved medical therapies offered by healthcare providers.
Of course, the Oregon Health Authority could change these rules. It will publish a final version of them by December 31, and various stakeholders have urged the agency turn Oregon’s program into something it is not. Some want Oregon facilitators to administer psychotherapy to clients as a treatment for mental health conditions. Others hope to turn the program into a statewide clinical trial to evaluate psilocybin as a drug therapy. But despite the desires of certain lobbyists, healthcare providers, and researchers to make Oregon’s program therapeutic and experimental, that’s not what Measure 109 does, and it’s not what Oregon voters approved.
Does that mean nobody will utilize Oregon’s services to treat their mental health conditions? Of course not. Some clients will seek psilocybin hoping to improve their mental health. The same could be said of many products and services, including yoga, massage, acupuncture, Tai Chi, meditation, exercise, and dietary supplements. But that doesn’t make these products and services medical therapies. Clearly, psilocybin has unique risks that are worth acknowledging and attempting to mitigate. But it’s important to understand what Oregon’s psilocybin law achieves. It allows people 21 years of age and older to receive psilocybin under supervision. It does not allow facilitators to play the roles of doctors, nurse practitioners, or therapists offering treatment for medical conditions.
That may not be the case for Colorado’s Proposition 122, the Natural Medicine Health Act. This law differs from Measure 109 in important ways. For instance, unlike Measure 109, Proposition 122 does not prohibit the governing agency from requiring clients to have medical diagnoses or a doctors’ prescriptions before receiving psilocybin. There are many other differences. For instance, there is no prohibition on situating service centers within healthcare facilities. Consequently, Colorado’s psilocybin program will not have the separation between healthcare services and psilocybin services, and the case report’s criticisms may be more applicable to Colorado than Oregon.
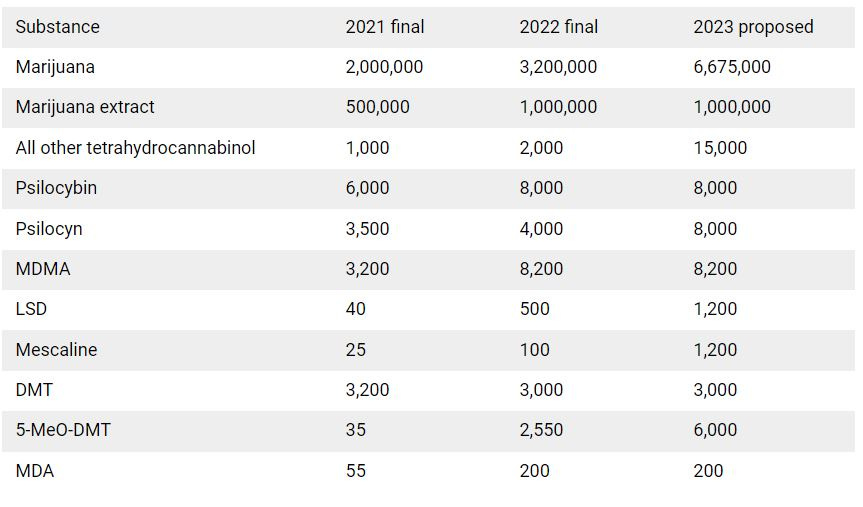
DEA raises national psychedelic production limits
In the 1970s, the federal Controlled Substances Act (CSA) placed most psychedelics on Schedule I of the controlled substances list, the most heavily restricted category. The Drug Enforcement Administration (DEA) enforces the CSA, and historically the agency has severely restricted nationwide production of psychedelics. The DEA’s limits have remained unchanged for years, contributing to the obstacles researchers face when studying Schedule I controlled substances. However, in recent years, the DEA has raised annual production limits in response to increasing demand. Last week, the agency announced its largest increase ever, dramatically raising limits on producing psilocybin, LSD, mescaline, and other psychedelics.
Equally notable was the DEA’s acknowledgement of the importance of psychedelics research. According to an agency notice, the “DEA is committed to ensuring an adequate and uninterrupted supply of controlled substances in order to meet the estimated legitimate medical, scientific, research, and industrial needs of the U.S., for lawful export requirements, and for the establishment and maintenance of reserve stocks.”
Portland’s pop-up mushroom shop
Last week, Willamette Week reported on Shroom House, Portland’s pop-up mushroom shop. On December 2, the paper described how a line of patrons wrapped around the block. Located on Portland’s busy West Burnside Street, the shop reportedly sells psilocybin mushrooms to customers for $40.
While waiting in line, patrons speculated when police might raid Shroom House, which ostensibly operates in defiance of state and federal drug laws. Oregon’s Measure 110 eliminated criminal penalties for possessing small amounts of psychedelics like psilocybin, and other controlled substances. However, sales and possession of large amounts remain illegal in Oregon. Meanwhile, Measure 109 legalized the supported adult use of psilocybin. But the Oregon Health Authority won’t issue licenses for psilocybin businesses until 2023, and according to Measure 109, only trained facilitators can administer psilocybin in licensed service centers.
Similar mushroom shops have been reported in Oakland, California and Toronto and Vancouver, Canada. On November 12, police raided a Toronto shop called Shroomyz. According to Willamette Week, the Oregon Health Authority directed questions regarding Portland’s Shroom House to local police. On December 2, the Portland Police Bureau told reporters it was aware of allegations regarding the shop but expected no further developments that day.
Some worry that illegal mushroom shops like Shroom House jeopardize the success of regulated psilocybin programs like Oregon’s and Colorado’s. They might also contribute to the existing backlash against Oregon’s Measure 110. Others view these shops as a welcome result of shifting attitudes toward psychedelics and a general loosening of restrictions.
Bioethicist responds to Breakthrough Therapies Act
On December 2, bioethicist Arthur Caplan and pharmaceutical entrepreneur Kenneth Moch published their reaction to the Breakthrough Therapies Act in STAT News. Senators Rand Paul and Cory Booker introduced the Act on November 17, with support from the Veterans Mental Health Leadership Coalition.
“Unfortunately, regulatory red tape and a series of bureaucratic hurdles involved in studying Schedule I substances impedes critical research on these and other promising Schedule I compounds,” said Senator Booker in a press release. “This bill will make it easier for researchers to conduct studies that can lead to breakthrough therapies to treat patients battling serious and life-threatening conditions,” added Senator Paul.
The bill would reduce barriers to studying substances in Schedules I and II for researchers authorized by a state to work with those substances. It would also make it easier to move substances from Schedule I to Schedule II if the Food and Drug Administration (FDA) designates them breakthrough therapies or they become available through its expanded use pathway.
Caplan and Moch are critical of the Breakthrough Therapies Act. They acknowledge that making Schedule I substances, such as psychedelics, available through expanded access could benefit some people. But because expanded access occurs outside of clinical trials, Caplan and Moch fear the Act might make it more difficult for researchers to enroll people in clinical trials, which could delay their completion and FDA approval of Schedule I substances. Their concern is legitimate, but the impact they describe is far from certain. Nevertheless, they raise another important point.
Under the Breakthrough Therapies Act, substances can only be rescheduled if they are actively being researched and commercialized. Specifically, the Act affects drugs that have been designated breakthrough therapies or are available through expanded access. The breakthrough designation requires completion of Phase I clinical trials, which evaluate safety in healthy volunteers, and expanded access requires an existing Investigational New Drug (IND) application. Researchers hoping to study Schedule I drugs that have not reached these milestones may encounter many of the same obstacles they faced before the Breakthrough Therapies Act.
That’s all true. However, making it easier to reschedule substances that have reached those milestones could be a significant improvement over the status quo. Currently, Schedule I substance must have completed at least Phase 2, and more likely Phase 3, clinical trials before being rescheduled. The Breakthrough Therapies Act would change that, and it’s difficult to see a downside of this rescheduling portion of the law. Even if substances are moved from Schedule I to Schedule II, they would still be heavily restricted, and most healthcare providers would be unable to prescribe them. The primary effect might be a modest to significant reduction in barriers to research. There might be better ways to reduce those barriers, but even a modest reduction would be better than none.
Caplan and Moch conclude with a critique of the dual-oversight framework created by the Controlled Substances Act (CSA), where the FDA and the Drug Enforcement Administration (DEA) share responsibility for regulating controlled substances. They ask, “[d]oes it make sense for two federal agencies to simultaneously be regulating the clinical development process for the same experimental medicine? We think not.” They’re right. But this criticism would apply to all controlled substances, not just those in Schedule I, and it seems like a critique of the CSA rather than an objection to the Breakthrough Therapies Act.
Caplan and Moch suggest that the FDA alone could “take on the oversight work to review how a Schedule I experimental medicine is being utilized and . . . review the release parameters for any Schedule I drug.” It’s not a bad suggestion. But under the CSA, the FDA already plays a prominent role, and significant barriers to research remain. Eliminating the DEA’s contribution could help, but it would be a significant departure from what the Controlled Substances Act currently requires, and the FDA may be no more supportive of research on Schedule I substances than the DEA.
For a detailed discussion of the Breakthrough Therapies Act, please join me on December 7 at 9:30 am Pacific/12:30 pm Eastern for an expert panel on Comparing Legal Approaches to Accessing Psychedelics. Hosted by the Project on Psychedelics Law and Regulation (POPLAR) at the Petrie-From Center at Harvard Law School.
Oregon Psilocybin Advisory Board’s final meeting of 2022
On Friday, December 9, the Oregon Psilocybin Advisory Board will hold its final meeting of 2022. In a recent article, Oregon Psilocybin Emails Show Secret Data Collection Plans, I described the secretive OPEN Project (now simply called OPEN) and the Oregon Health Authority’s plan to host a discussion on data collection during the Advisory Board’s December meeting. However, following the article’s publication, the Oregon Health Authority cancelled this discussion.
In the agenda for the upcoming meeting, data collection appears only in a list of miscellaneous ideas at the end of the Board’s Strategic Plan Working Document. The Board will discuss this working document for approximately ninety minutes. It is unclear how much time, if any, will be devoted to discussing data collection. One of the assorted topics listed is the creation of a “mechanism to collect and analyze client-level data and integrate learning” into the Board and Health Authority’s work. For more on the risks of mandated data collection, see Seeking Psychedelics? Check the Data Privacy Clause.
Upcoming events
Disclosed: Conflicts of Interest in the Psychedelic Ecosystem
December 6 at 12 pm Pacific/3pm Eastern. Hosted by the Project on Psychedelics Law and Regulation (POPLAR) at the Petrie-Flom Center at Harvard Law School, in collaboration with the Chacruna Institute.
Comparing Legal Approaches to Accessing Psychedelics
December 7 at 9:30 am Pacific/12:30 pm Eastern. Hosted by the Project on Psychedelics Law and Regulation (POPLAR) at the Petrie-Flom Center at Harvard Law School.
*The views expressed on Psychedelic Week do not represent the views of POPLAR at the Petrie-Flom Center at Harvard Law School or the Florida State University College of Law. Psychedelic Week is an independent project unaffiliated with these programs and institutions.
Mason Marks, MD, JD is the Florida Bar Health Law Section Professor at the Florida State University College of Law. He is the senior fellow and project lead of the Project on Psychedelics Law and Regulation (POPLAR) at the Petrie-Flom Center at Harvard Law School and an affiliated fellow at the Information Society Project at Yale Law School. Marks teaches constitutional law, administrative law, drug law, and psychedelic law, . Before moving to Florida, he served on the Oregon Psilocybin Advisory Board where he chaired its Licensing Subcommittee. Marks has drafted drug policies for state and local lawmakers. His forthcoming book on psychedelic law and politics will be published by Yale University Press. He tweets at @MasonMarksMD and @PsychedelicWeek.